
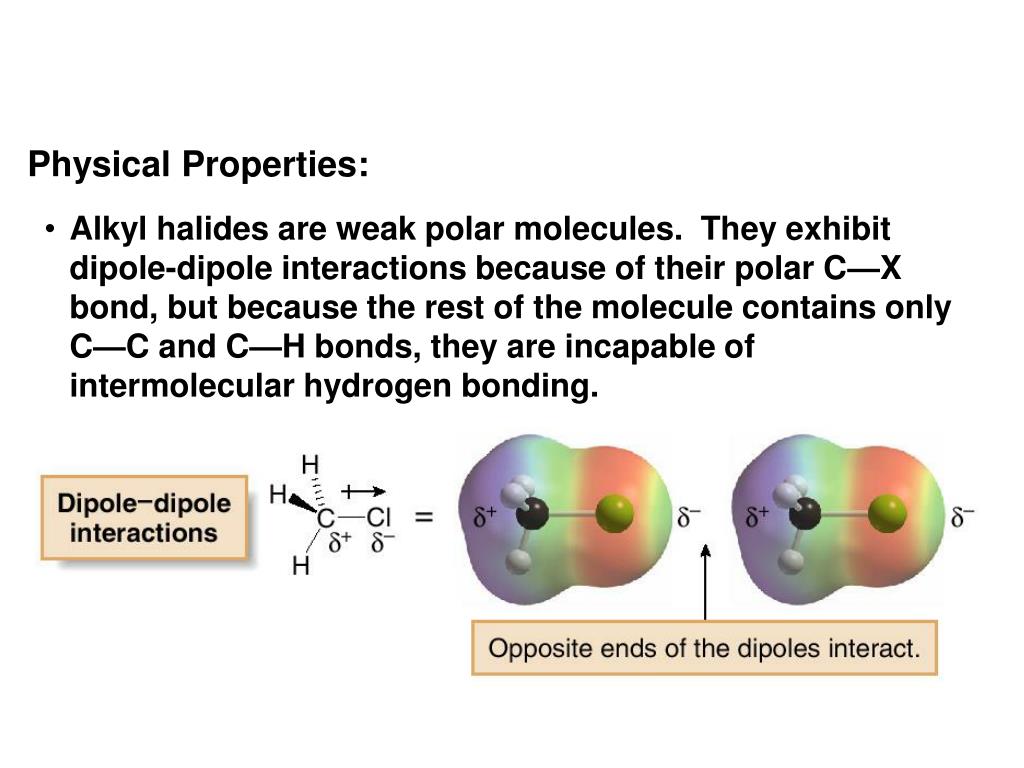

This review summarises the development of these host systems to date, by first examining the various neutral and cationic XB donor motifs commonly employed and their integration into supramolecular acyclic and macrocyclic receptors. The halogen atoms differ greatly in size and charge from hydrogen and from each other. These XB receptors have contrasting, and often superior anion binding affinities and properties when compared to their analogous hydrogen bonding receptors. The nonbonding electrons donated are used to form unsaturated bonds between the halogen and the substrate molecule. In the latter, a hydrogen atom is shared between an atom, group or molecule that donates and another that accepts it. Halogen atoms are the electronegative atoms which have lone pair of electrons that can be donated to the substrate molecule.
The use of halogen bonding (XB) in anion recognition chemistry has rapidly expanded in recent decades, with a diverse range of host systems available for the selective complexation and detection of various anionic guest species in solution. What is halogen bonding Halogen bonding (XB) is a noncovalent interaction that is in some ways analogous to hydrogen bonding (HB). Group 7A (or VIIA) of the periodic table are the halogens: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). The halogen bond is a powerful non-covalent interaction between the electron deficient sigma-hole region of a group 17 halogen element covalently bonded to electron withdrawing groups, and a Lewis base.


 0 kommentar(er)
0 kommentar(er)
